Size Exclusion Chromatography (SEC) is a commonly used method for extracellular vesicle (EV) and exosome isolation from biological fluids. SEC is easy, reproducible, and provides a high yield of purified EV’s that retain their functionality (Table 1). Furthermore, the minimal information for studies of extracellular vesicles (MISEV2023) guidelines highlights SEC as a very accessible size-based technique for separation of EVs from non-vesicle associated extracellular proteins. These advantages are propelling SEC to becoming one of the most widely used EV isolation techniques.
Unfortunately, SEC is low throughput when performed manually. This has limited its utility for biomarker discovery and diagnostic research until recently.
| Isolation Method | Reproducibility | Ease of Use | Purity | Yield |
| SEC | +++ | +++ | ++ | +++ |
| Precipitation | ++ | +++ | + | +++ |
| Ultracentrifugation | + | ++ | + | + |
| Density Gradient | ++ | + | +++ | + |
Table 1: Performance comparison between the most used purification techniques for EVs and exosomes1,2.
How does SEC purify EVs?
SEC separates particles and biomolecules based on size. EVs size range is around 30-200 nm, while contaminant molecules and particles range from 7-68 nm3. In a SEC column, EVs are separated from smaller particles as they flow through porous Sepharose resin beads. Most contaminants in biological fluids (e.g. albumin, immunoglobulins, HDL, and LDL) are smaller than the pore size, so they travel slower as they traverse through the resin pores and elute later (Figure 1). Sepharose resin is available with different amounts of agarose, which results in different pore sizes (Table 2). This allows columns to be optimized with different EV purity and yields3,4. Sepharose CL-6B and CL-4B allow EVs across the expected size range to elute in the void volume of the column. The larger pore size of Sepharose CL-2B is sometimes used to further deplete larger lipoprotein particles e.g., IDL and VLDL. This additional purity comes with significant loss of EVs that limit its use for the characterization of low-abundant EV subpopulations5,6.
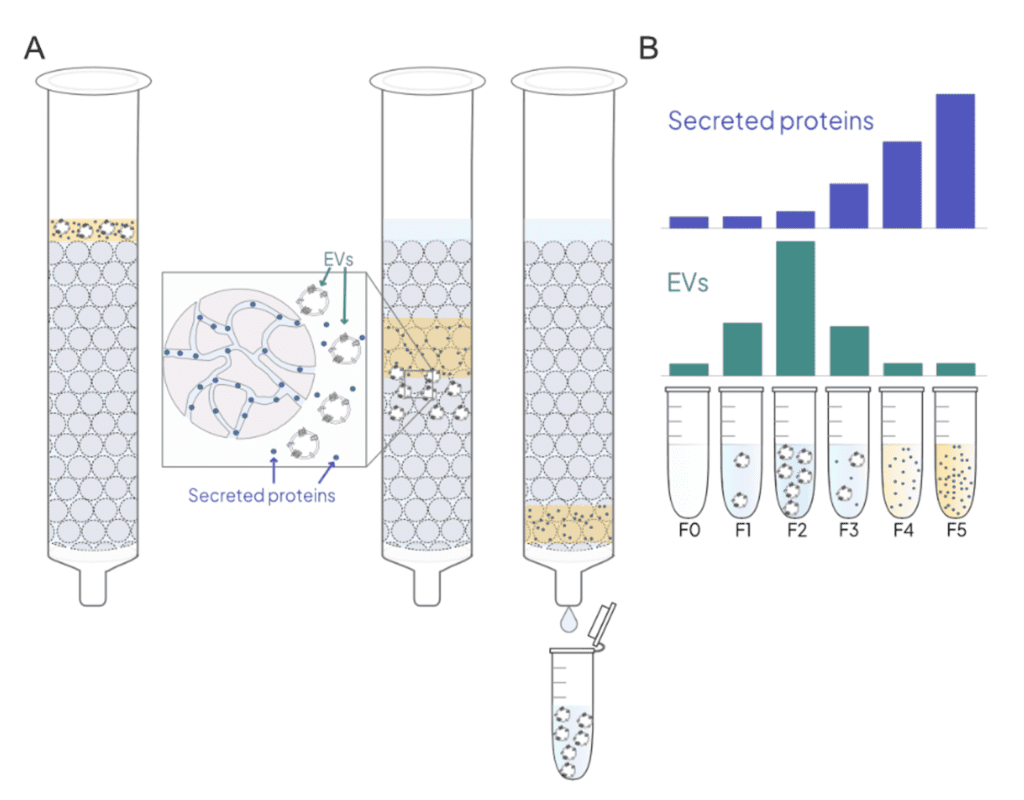
Figure 1: A. Schematic showing the principle of SEC. B. Typical elution profile of EVs and secreted proteins after SEC fractionation
| Resin Type | Agarose amount | Exclusion limit |
| Sepharose CL-2B | 2% | 75 nm |
| Sepharose CL-4B | 4% | 35 nm |
| Sepharose CL-6B | 6% | 20 nm |
Table 2: Specification of cross-linked Sepharose resins
How to speed-up and standardize EV isolation with Everest Biolabs solutions?
Apex 6B Column: Sepharose CL-6B column optimized for the highest EV recovery. Ideal for targeted downstream analysis including immunoassays, label-based imaging assays, label-based flow assays, western blots, and pull-down using a specific surface marker. Learn more
Apex 4B Column: Sepharose CL-4B column optimized for the highest purity. Ideal for unbiased downstream analysis, including proteomics and RNA-seq. Learn more
Ascent Instrument: Automated, high-throughput EV isolation with SEC columns. Learn more